Highlights
- Off-site follow-up of trial participants by use of digital technologies
- Improved recruitment by reducing the burden for trial participants
- Validated and novel digital endpoints
- One-stop-shop: From trial design and execution to data analytics
- Both hybrid and fully virtual trial services possible
Summary
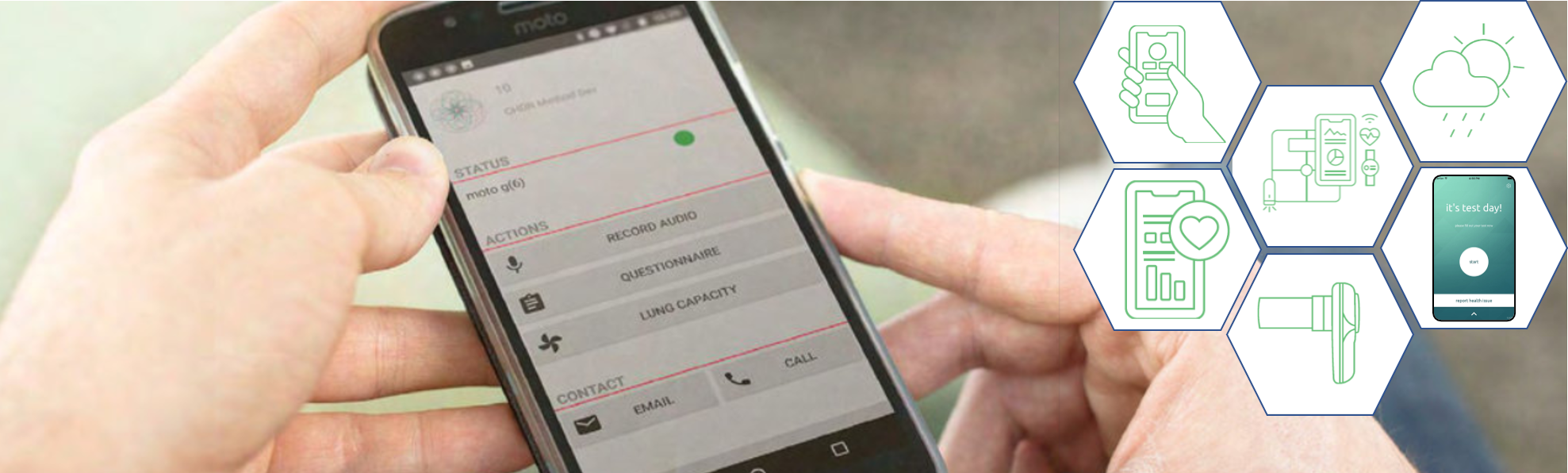
Establishing that medicines work, in the sense that they influence a certain pathophysiological mechanism, is a lot easier than demonstrating that they help. To show that they help, it is crucial to include value-based evaluation. This requires follow up of patients who do not necessarily have events of a condition (e.g. a stroke). Trial@home enables this by using technology and assuring that data is reliably collected while trial participants are not in the clinic.
What Trial@home offers
With more than 30 years of experience in designing and executing clinical trials, CHDR assures that your trial is designed to the highest scientific standards.
Data analytics servicesNon‑clinic data require a different data analytics approach. With in‑house expertise in Artificial Intelligence and biological signal processing, CHDR assures that all information in the collected data is extracted for a better evaluation of the trial outcomes.
Validated and novel digital endpoints
With a scientific background, CHDR assures that all novel digital endpoints that are developed are properly validated with a rigorous scientific approach.
Seamless integration with digital technologyDigital technology has been emerging in the healthcare and drug development world. CHDR is constantly looking for candidate technologies to be integrated in our custom‑built platform. Thorough validation of the technology is performed (in terms of relevance of the measure, data quality and transparency, ease of use for trial participants) before integration.
Integration with our Electronic Data Capturing system (Anju Promasys)To ease transfer of data, we have full integration with our EDC system that allows endpoints to be ready for statistical analysis and transfer as soon as possible.