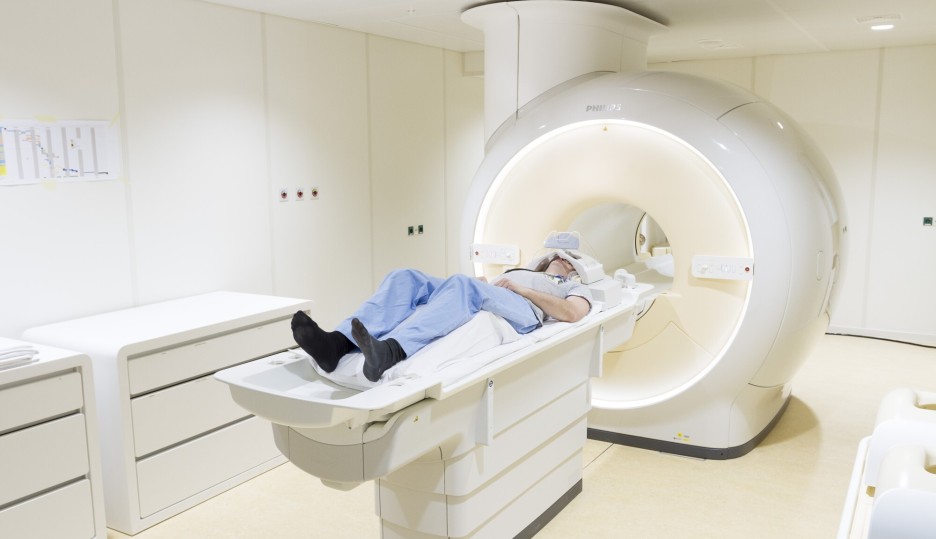
Magnetic Resonance Imaging (MRI)
At CHDR, we utilise Resting-State functional MRI (RS-fMRI) to study the effects of compounds targeting the central nervous system (CNS). RS-fMRI is a non-invasive, high-resolution imaging technique that allows us to monitor brain activity and connectivity without requiring specific tasks. This method is particularly valuable for assessing drug effects on the brain, even in small subject groups, with high sensitivity.
We collaborate with the Pharmaco-MRI Research Unit of the Leiden University Medical Center (LUMC) for MRI, utilising their dedicated 3-tesla MI scanner, which is exclusively reserved for research purposes. This arrangement ensures excellent availability and short turnaround times, without interfering with patient care.
We have developed a library of RS-fMRI ‘fingerprints’ for well-characterised psychoactive compounds through extensive research and collaboration with our partners. This enables us to track changes in brain function and connectivity in response to various drugs. Additionally, RS-fMRI can be combined with task-based fMRI and other CNS function tests, such as NeuroCart® and PainCart®, for a comprehensive assessment of CNS activity and therapeutic impact.
Positron Emission Tomography (PET)
We use Positron Emission Tomography (PET) imaging to gain valuable insights into how test compounds interact with specific molecules in the body, bridging preclinical and early clinical drug development. Our expertise allows us to visualise drug distribution and binding in target tissues, enabling precision pharmacology by combining PET with pharmacokinetics (PK) and pharmacodynamics (PD) modelling.
We also integrate PET imaging with functional CNS testing, electrophysiology assessments, and tools like NeuroCart® and PainCart® to provide a comprehensive view of drug effects, enhancing our ability to understand the mechanisms of action and therapeutic potential of novel compounds.
FDG-PET
Fluorodeoxyglucose Positron Emission Tomography (FDG-PET) can be used to assess glucose metabolism in tissues, providing valuable insights into cellular activity and disease processes. By using FDG, a radiolabelled glucose analogue, FDG-PET enables the non-invasive visualisation of metabolic activity in various organs, particularly the brain. This is especially useful for studying neurological conditions, cancer, and metabolic disorders.
FDG-PET can be combined with other imaging techniques, pharmacokinetic studies, and biomarker analysis to offer a comprehensive understanding of drug effects, disease progression, and therapeutic response.
Magnetic Resonance Spectroscopy (MRS)
We utilise Magnetic Resonance Spectroscopy (MRS) to gain detailed insights into the metabolic composition of tissues, particularly in the brain and muscles. MRS allows us to non-invasively measure the concentration of specific metabolites, providing valuable information on brain function, disease processes, and therapeutic effects. This technique is especially useful for studying neurodegenerative disorders, brain tumours, and the effects of new treatments on brain metabolism.
In addition to brain studies, we also use MRS for muscle mitochondrial function measurements, as well as assessing mitochondrial function in the occipital cortex. Furthermore, we can measure phosphocreatine recovery time, a key indicator of muscle metabolic efficiency
Deuterium-based myelin labelling
Deuterium-based myelin labelling can be used to study myelin synthesis and turnover in the central nervous system (CNS). By incorporating deuterium, a stable isotope of hydrogen, into the lipid molecules of myelin, we can track the incorporation of newly synthesised myelin over time. This technique provides detailed insights into the dynamics of myelin formation, its renewal processes, and the effects of therapeutic interventions on myelin health.
Deuterium-based labelling offers a non-invasive way to investigate myelin turnover, which is critical for understanding neurodegenerative diseases, demyelinating conditions like multiple sclerosis, and the impact of CNS-targeted treatments. Through this advanced labelling approach, CHDR provides valuable data to support drug development and clinical research aimed at preserving or repairing myelin.
Hyperspectral retinal imaging
Hyperspectral retinal imaging is an advanced technique that provides a detailed view of the retina by capturing a wide range of light wavelengths, beyond the visible spectrum. This enables the analysis of tissue composition, oxygenation, and metabolic changes with high precision. By detecting subtle differences in light absorption, hyperspectral imaging allows for the identification of early pathological changes in retinal tissue.
This method is particularly useful for studying retinal conditions such as diabetic retinopathy, age-related macular degeneration, and other retinal diseases. It offers a non-invasive way to monitor disease progression and assess the effects of therapeutic interventions, providing valuable insights for clinical research and drug development.
Image-guided surgery
We have a unique collaboration with Leiden University and the Leiden University Medical Center (LUMC) in the field of Image-Guided Surgery (IGS). Working together, the IGS team develops powerful image-guided approaches for use in surgery, diagnostics, and other clinical applications. The combined effort of surgical oncologists and CHDR’s world-class drug-development expertise opens up exciting new opportunities, bringing important medical breakthroughs to clinical practice.