At CHDR, we specialise in data-intensive, methodologically rich early-phase clinical pharmacology studies, working with both healthy participants and small patient cohorts. Our expertise extends to the field of (immuno-)oncology, where we offer a comprehensive suite of services. Leveraging our central position within a large academic network, we combine advanced capabilities, including a bedside laboratory with cell culture facilities, a fully validated flow cytometry unit, and a proprietary set of ex vivo and in vivo models designed to drive controlled damage, inflammation, and immune responses.
Furthermore, CHDR collaborates closely with the esteemed Dutch Cancer Institute (NKI) in Amsterdam, renowned for its dedication to advancing cancer research and clinical applications. This partnership ensures that we remain at the forefront of translating innovative basic research into tangible clinical solutions.
Solutions and capabilities
Cellular function monitoring and assays
CHDR offers a comprehensive suite of in-house developed assays to monitor cellular function, using circulating leukocytes to assess drug effects. These assays are conducted on fresh human whole blood or PBMCs, with the flexibility to extend to other matrices such as bone marrow or skin explants. When necessary, cells are exposed to stimuli that activate stress pathways relevant to the investigational drug. Validated endpoints encompass key biological processes, including cytokine release, cell activation and proliferation, mitochondrial function, lysosomal function, and cell-metabolic signaling.
In vivo human immune challenge models
CHDR is a world leader in the development of in vivo human immune challenge models, which are the gold standard for early clinical evaluation of investigational drugs targeting autoimmune or inflammatory diseases. These models are equally applicable for evaluating new classes of immuno-oncological therapies. Our in vivo human challenge models include, but are not limited to, intravenous and intradermal lipopolysaccharide (LPS) challenges to assess systemic and local immune responses, exposure to neoantigen KLH for controlled induction of primary and recall antigen responses, and damage-based immune challenges such as skin UVB exposure and controlled wound infliction via skin punching to assess tissue repair and immune activation and resolution. These models drive key physiological processes, including immune and inflammatory responses, (micro)vascular reactions, tissue repair, and leukocyte trafficking. CHDR has extensively characterised these models at the transcriptomic and proteomic levels and offers robust data mining of these datasets to identify druggable oncological targets (image 1). In addition, our team has the scientific and technical expertise to customise existing models or co-develop novel in vivo challenge models that align precisely with the unique mechanisms and therapeutic goals of cutting-edge immuno-oncology treatments.
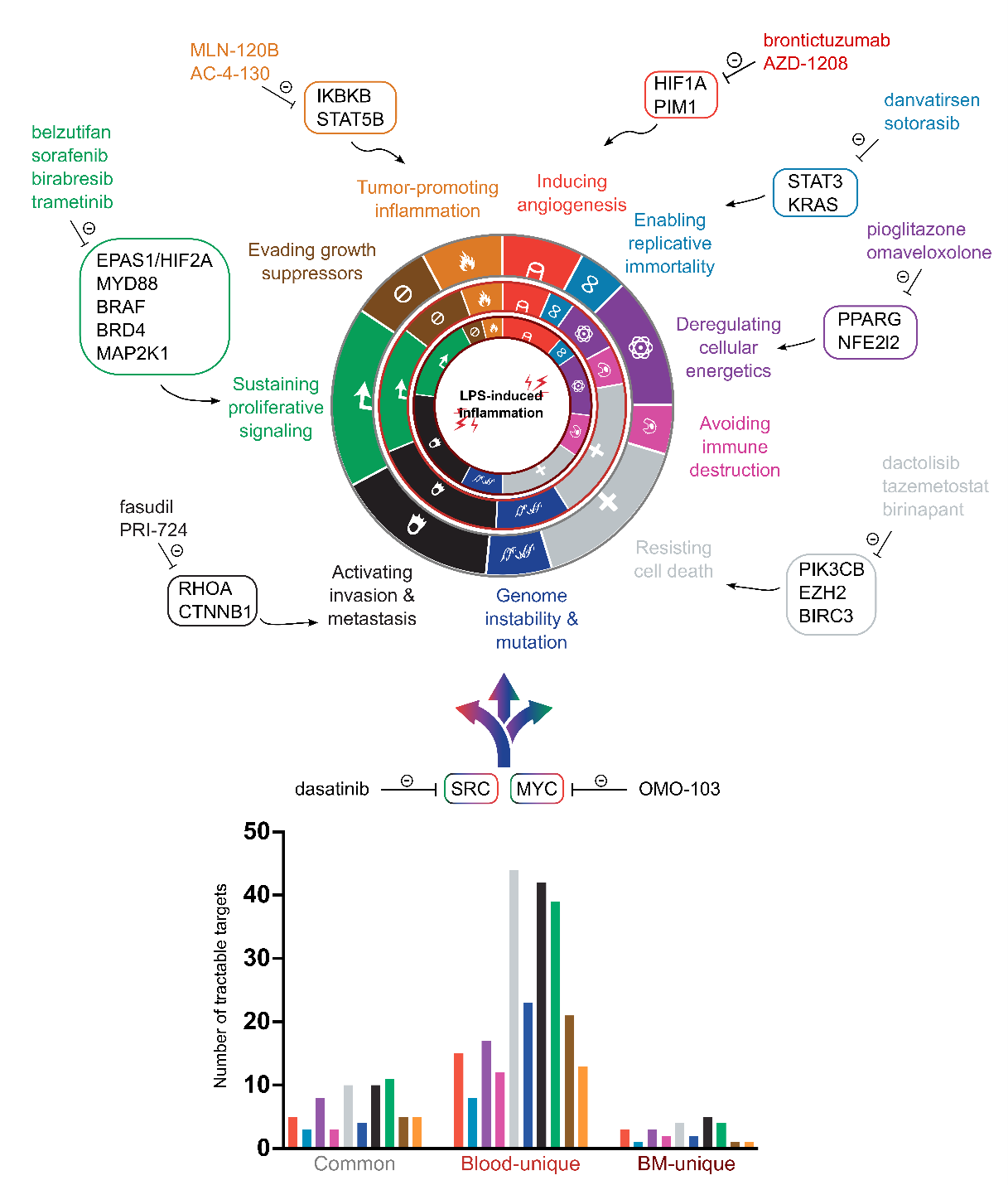 Image 1. Cancer hallmarks gene expression in blood and bone marrow after systemic (intravenous) LPS challenge in healthy participants. Understanding where and how oncology targets are expressed is crucial. This figure maps a selection of druggable targets to cancer hallmarks, comparing their upregulation in peripheral blood versus bone marrow. See common targets (outer circle), blood-specific (middle circle), and bone marrow-specific (inner circle) expression patterns, along with example drugs. Our analysis identifies targets relevant across multiple hallmarks, such as SRC and MYC, and quantifies targets per category (bar chart), which provides insights for target and biomarker validation in IO drug development.
Image 1. Cancer hallmarks gene expression in blood and bone marrow after systemic (intravenous) LPS challenge in healthy participants. Understanding where and how oncology targets are expressed is crucial. This figure maps a selection of druggable targets to cancer hallmarks, comparing their upregulation in peripheral blood versus bone marrow. See common targets (outer circle), blood-specific (middle circle), and bone marrow-specific (inner circle) expression patterns, along with example drugs. Our analysis identifies targets relevant across multiple hallmarks, such as SRC and MYC, and quantifies targets per category (bar chart), which provides insights for target and biomarker validation in IO drug development.
Omics-based endpoints and integrated workflow
CHDR provides a fully integrated workflow for omics-based endpoints, collaborating with highly qualified partner labs for transcriptomic, proteomic, and metabolomic analysis of human samples. With expertise in collecting and handling non-standard specimens such as bone marrow, skin biopsies, and cerebrospinal fluid, CHDR ensures the highest quality sample processing. We also offer in-house bioinformatics support to analyse and interpret large omics-based datasets, providing actionable insights for drug development.
Case studies
-
Adverse immunostimulation is a common side effect of oncological drugs. For compounds like paclitaxel and doxorubicin, unintentional activation of the complement system can lead to hypersensitivity reactions. CHDR, in collaboration with the Dutch Cancer Institute (NKI), has worked to deepen the understanding of drug-induced hypersensitivity in patients with breast or ovarian cancer, aiming to improve patient safety and treatment outcomes.
-
CHDR has extensive experience in the early clinical evaluation of 'high-risk' compounds in healthy participants. Examples include the investigation of IL-2 muteins or lymphocyte-depleting antibodies. When necessary, studies are conducted at our LUMC unit under intensive medical supervision. After establishing the initial pharmacokinetic and pharmacodynamic relationships for a range of low doses in healthy participants, we transition to small cohorts of patients. These investigations can be conducted under a single clinical study protocol, ensuring streamlined and efficient progression.
-
CHDR is a frontrunner in deep phenotyping of dermatological and gynecological conditions. Our clinical studies have explored treatments for conditions such as cutaneous T-cell lymphoma and various gynecological disorders, providing invaluable insights into the mechanisms underlying these conditions and supporting the development of targeted therapies.
-
CHDR invests in method development to improve the mechanistic profiling of registered drugs. One such example is the evaluation of the c-KIT inhibitor imatinib in healthy participants, with a focus on microvascular function and leukocyte signalling. This study involved collecting blood, bone marrow punctures, and skin biopsies to better understand the compound's effects and inform future therapeutic strategies.