At CHDR, we consider the failure of most innovative treatments to reach patients as a fundamental challenge that must be addressed. Psychiatry, in particular, urgently needs more effective, faster-acting drugs with minimal side effects. In early-phase clinical trials, the traditional focus on pharmacokinetics and safety, and the insufficient focus on demonstrating proof-of-pharmacology (PoP), contribute to the relatively high failure rates in psychiatric drug development. Currently, only approximately 6% of novel drugs entering phase 1 trials ultimately make it to the market.
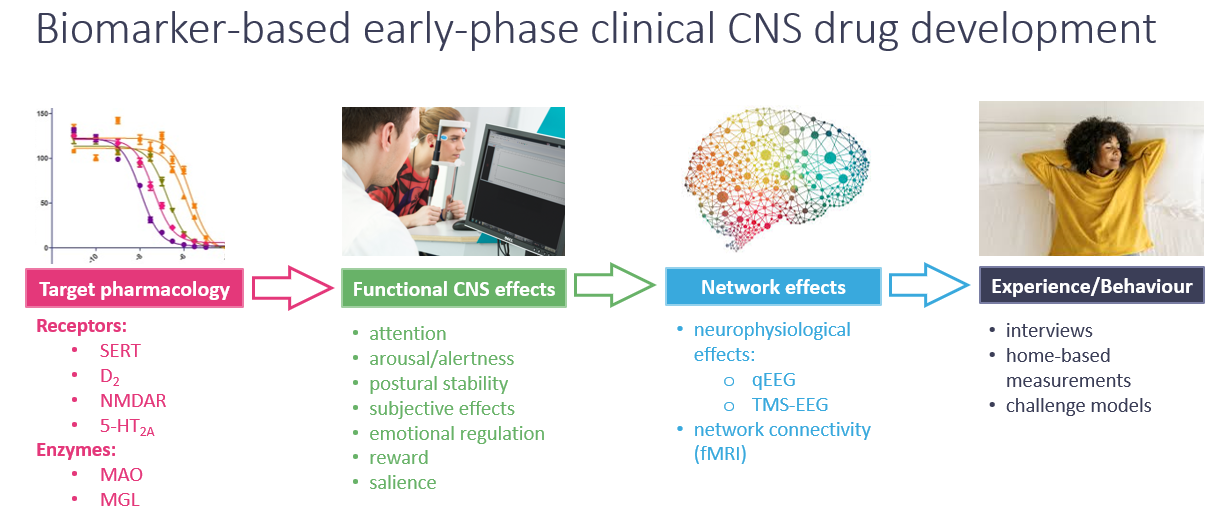
We therefore strongly advocate for biomarker-driven early drug development, which encompasses PoP in healthy volunteers, as well as proof-of-mechanism (PoM) and proof-of-concept (PoC) in patient populations. At CHDR, we propose an innovative approach to early drug development that is guided by the functional organisation of the central nervous system (CNS). By understanding CNS function — from molecular interactions and functional effects to network adaptation, behavioral outcomes, and changes in subjective experience — our approach ensures thorough pharmacological characterisation of novel compounds. This process helps to mitigate risks, such as lack of efficacy or unexpected safety in later stages of development:
Solutions and capabilities
CHDR uses a biomarker-based approach to psychiatric drug development, focusing on pharmacodynamic effects on drug-sensitive CNS functions in humans. This aligns with preclinical research aiming to link CNS function to pharmacology. We view CNS target engagement as an essential first step in a cascade of neurophysiological events that lead to changes in behaviour, cognition, and emotional regulation. By using drug-sensitive biomarkers in early-phase trials with healthy volunteers and patients, we develop novel compounds and optimise biomarkers that track drug exposures, minimise test-retest variability, and reduce volunteer burden.
Neuro- and PsyCart
Our NeuroCart® CNS test battery has been crucial in advancing CHDR’s biomarker-based approach to early drug development. It assesses the pharmacological effects of compounds on a range of neurophysiological and neuropsychological functions, including arousal, attention, motor function, psychomotor stability, and alertness. CHDR has characterised the pharmacodynamics of hundreds of novel compounds, building an extensive database of "pharmacological fingerprints" for benchmarking in first-in-human (FIH) studies. Efforts are underway to expand NeuroCart by integrating a new test battery focused on functional CNS domains relevant to psychiatric drug development, including reward processing, arousal, salience, and emotional processing, based on the United States National Institute of Mental Health’s (NIMH) Research Domain Criteria (RDoC) initiative.
Challenge models
Drug effects may not be evident under non-challenged physiological conditions, especially in healthy participants, due to floor or ceiling effects. To address this, CHDR has developed and optimised various challenge paradigms to demonstrate drug effects related to specific MoAs in healthy subjects. Examples include esketamine and biperiden models, inducing psychosis-like effects and cognitive dysfunction, respectively. These models help evaluate potential antipsychotic or pro-cognitive effects. Additionally, CHDR’s CO2 inhalation challenge induces short-lived panic attacks, providing a controlled setting to assess novel compounds for emotional hyperarousal reduction or anxiolysis. Read more here.
Sleep
Sleep disorders often co-occur with psychiatric conditions, but many sedative-hypnotics negatively affect sleep architecture and next-day cognitive or psychomotor function. This highlights the need for novel CNS-penetrant compounds that improve sleep without these drawbacks. CHDR evaluates such compounds by quantifying sleep architecture with polysomnography (PSG) and assessing subjective sleep through patient-reported outcomes. Additionally, the impact of these compounds on cognition and psychomotor function is assessed using the NeuroCart and the Green Dino Driving simulator, providing valuable insights into drug safety and performance.
Further capabilities
Additional capabilities relevant to early psychiatric drug development include CSF sampling to confirm blood-brain barrier penetration, EEG, TMS-EEG, and various neuroimaging techniques, including resting-state fMRI and PET, which can be applied to confirm target engagement and/or relevant changes in brain connectivity networks.
Psychedelic therapies
A paradigm shift is happening in psychiatry with novel compounds that offer sustained therapeutic effects after only a few doses, unlike traditional treatments requiring daily administration. These include serotonin 5-HT2A receptor agonists like DMT, psilocybin, and MDMA, as well as NMDAR antagonists like ketamine. These compounds, linked to enhanced synaptic plasticity, may also be combined with psychotherapy for better outcomes.
CHDR provides comprehensive pharmacological characterisation of these compounds in early-stage development. By combining validated psychedelic effect assessments with the NeuroCart test battery and EEG, we evaluate their pharmacodynamic effects on key CNS functions, linking these to desired and undesired outcomes.
With expertise in 5-HT2A receptor agonists and NMDAR antagonists, CHDR benchmarks novel compounds against established ones like ketamine and DMT. Our team, trained by leading experts in psychedelic research, is committed to maintaining rigorous scientific standards while ensuring participant safety and wellbeing, by supporting them through what can be intense psychological experiences.
Patient studies
CHDR is committed to upholding the highest medical ethical standards throughout the recruitment process and obtaining informed consent from all patients. In addition to adhering to ethical rigor, CHDR implements a reliable and efficient recruitment strategy, which involves close collaboration with patients' general practitioners, attending psychiatrists, and psychotherapists. This approach not only ensures the continuity of ongoing care but also guarantees the safety, well-being, and support of participating patients throughout the study.
Over the past decade, CHDR has successfully conducted a wide array of proof-of-concept (PoC) studies involving novel CNS compounds across diverse patient populations, spanning a broad spectrum of psychiatric disorders. These studies have encompassed conditions such as major depressive disorder (MDD), treatment-resistant depression (TRD), and persistent depression/dysthymia, in addition to various anxiety disorders, trauma-related conditions including PTSD, and sleep disorders.
Case studies
-
The 5-HT2AR agonist N,N-dimethyltryptamine (DMT) has shown promising therapeutic potential for conditions like major depressive disorder (MDD), post-traumatic stress disorder (PTSD) and addiction-related disorders. However, before its therapeutic effects in psychiatric conditions can be fully explored, dedicated clinical pharmacology studies are necessary. These studies will help clarify the relationship between DMT’s PK and its PD effects, identify potential sources of PK variability, and establish optimal DMT infusion regimens for combination with psychotherapy.
In this context, CHDR conducted a trial with healthy participants to investigate the safety, PK and PD of DMT administered through different intravenous (IV) regimens. The study found that DMT was safe when administered IV, either as a bolus, a bolus followed by an infusion, or as a continuous infusion, all reaching the expected psychedelic exposure threshold. Measurements were performed repeatedly and closely tracked the respective pharmacokinetic profiles of each DMT administration regimen, enabling us to delineate the evolution of CNS effects over time, thereby setting the stage for PK-PD modelling to inform the design of future clinical studies with IV DMT.
While previous studies involving 5-HT2AR agonists, including DMT, have combined subjective psychedelic drug measures with EEG and/or fMRI, this study, to our knowledge, is the first to link various IV DMT infusion regimens to their respective PK profiles on the one hand, and not only subject psychedelic drug measures but these combined with functional CNS assessments using NeuroCart, EEG, neuroendocrine and cardiovascular effects, on the other hand. Moreover, this approach provided valuable insights into the reliability of different PD assessments for quantifying the effects of psychedelic compounds, opening avenues to potential novel pharmacological biomarkers for 5-HT2AR agonists in future early-stage drug trials.
-
CHDR has supported the early development of the novel, brain-permeable, selective ATP-gated P2X7 purine receptor (P2X7R) antagonist JNJ-54175446. P2X7R antagonists are currently in development for the treatment of major depressive disorder (MDD) and bipolar disorder. In preclinical studies, JNJ-54175446 attenuated dexamphetamine-induced hyperlocomotion in rodents.
In the single ascending dose (SAD) study, JNJ-54175446 was shown to be safe, cross the blood–brain barrier, and demonstrate a dose–receptor occupancy relationship as observed through positron emission tomography (PET).
ATP concentrations in the healthy human brain were anticipated to be too low to significantly activate P2X7Rs. CNS effects resulting from P2X7R antagonism were therefore only expected following ATP release, and as a result, no or limited pharmacodynamic (PD) effects were anticipated in an unchallenged, healthy volunteer population. To address this, in the multiple ascending dose (MAD) study, dexamphetamine was administered as a potential translational pharmacological challenge, as it had been shown to not only increase hyperlocomotion in rodents, but also demonstrated a psychostimulatory profile in healthy participants on NeuroCart. Similar to preclinical findings, JNJ-54175446 attenuated dexamphetamine-induced stimulatory CNS effects, suggesting potential mood-modulating effects in humans.
The successful attenuation of dexamphetamine-induced CNS effects in healthy volunteers indicated that acute mood disruption is necessary to demonstrate P2X7R antagonism in humans. Therefore, a proof-of-concept study was performed in MDD patients to evaluate potential mood-modulating effects in a clinically relevant population. Given ethical concerns around administering dexamphetamine to MDD patients, total sleep deprivation (TSD) was used as an alternative mood-disrupting intervention TSD has been shown to acutely but transiently improve mood in MDD patients, allowing the investigation of potential mood modulation by JNJ-54175446. While JNJ-54175446 did not have a significant effect on self-reported mood, it successfully blunted TSD-induced reduction of anhedonia, suggesting a potential mood stabilising effect, rather than a traditional antidepressant effect, in patients with mood disorders.
Since ATP concentrations in the healthy human brain are typically too low to significantly activate P2X7Rs, it was expected that the compound’s pharmacodynamic (PD) effects would only emerge under conditions of ATP release or activation. Consequently, no significant PD effects were anticipated in healthy volunteers under normal conditions.
To address this, the multiple ascending dose (MAD) study introduced dexamphetamine as a pharmacological challenge, which had been shown to induce hyperlocomotion in rodents and exert a psychostimulatory effect in healthy participants, as measured by NeuroCart. As anticipated, JNJ-54175446 attenuated dexamphetamine-induced stimulatory CNS effects, suggesting potential mood-modulating effects in humans.
-
5-HT2AR agonists have recently emerged as promising candidates for the treatment of neuropsychiatric disorders, with their therapeutic effects hypothesized to be mediated by the promotion of structural neuroplasticity. However, their hallucinogenic properties and consciousness-altering effects may limit their broad clinical application and scalability. This has driven industry interest in developing non-hallucinogenic analogues, sometimes classified as ‘neuroplastogens’.
CHDR conducted a first-in-human (FIH) study with the novel non-hallucinogenic neuroplastogen DLX-001, with the aim of assessing its safety, pharmacokinetics, and pharmacodynamic profiles. Building on our extensive experience with various “classic” 5-HT2AR targeting psychedelic compounds, we demonstrated that DLX-001 lacked either hallucinogenic properties or untoward functional CNS effects on subject psychedelic drug measures and the NeuroCart test battery, respectively. At the same time, we confirmed CNS penetration and target engagement by demonstrating relevant drug exposures in cerebrospinal fluid (CSF), which were associated with neuroendocrine hormone release and EEG changes similar to those seen in major depressive disorder (MDD) patients responding to electro-convulsive therapy (ECT).
This study highlights the added value of CHDR’s question-based drug development approach, using appropriate pharmacodynamic biomarkers to inform the early development of DLX-001.
-
GABA-A receptor (GABA-A-R) α2/3/5-subtype selective positive allosteric modulators (PAMs) enhance inhibitory neurotransmission while avoiding α1-subtype mediated effects. This selectivity offers the potential for anxiolytic effects without the sedative, psychomotor, and cognitive impairments typically seen with non-selective GABA-A-PAM benzodiazepines.
Using NeuroCart and EEG, CHDR has profiled numerous CNS-active compounds that facilitate GABAergic neurotransmission in humans. NeuroCart successfully differentiated the pharmacodynamic profiles of non-selective GABA-A-R PAMs, such as alprazolam and lorazepam, from the novel GABA-A-R α2/3-subtype-selective PAMs, including TPA023, MK-409, SL65.1498, NS11821, AZD7325 and AZD6280 (see thesis 1 and thesis 2). Non-selective compounds consistently reduced saccadic peak velocity (SPV), visual analogue scale (VAS) alertness, and adaptive tracking, while increasing body sway. In contrast, α2/3-subtype selective compounds reduced SPV to a comparable extent at equipotent doses, but demonstrated minimal or no effects on VAS alertness, adaptive tracking, and body sway. Regression analysis further highlighted distinct profiles between selective and non-selective PAMs, particularly in the relationship between SPV change from baseline and body sway, adaptive tracking and VAS alertness.
CHDR recently performed a multiple ascending dose (MAD) study with the novel GABA-A α2/3/5-subtype selective PAM ENX-102 to characterise its safety, pharmacokinetics, and pharmacodynamic characteristics following single and multiple doses. GABA-A-R α2/3/5 target engagement was confirmed by reductions in SPV, indicating reduced arousal after a single dose. Although single-dose SPV reductions were associated with undesired CNS effects, such as reduced sustained attention, the reduction in SPV was sustained following multiple dosing, with the undesired effects dissipating. Importantly, no sedation was reported following either single or multiple dosing. These findings support the selective targeting of α2/3/5 subunits, avoiding α1 subunit activation, and provide preliminary evidence for the attenuation of behavioural states associated with heightened arousal and/or cortical hyperexcitability—such as anxiety, trauma-related disorders, or epilepsy— via selective GABA-A-R α2/3/5 subtype agonism.
This study highlights the importance of detailed pharmacological profiling of novel CNS-penetrant compounds using a reliable biomarker platform such as NeuroCart in early drug development. The insights gained from this study played a key role in advancing ENX-102 into the Phase 2 ENCalm study, which investigates its potential as a monotherapy for generalised anxiety disorder (GAD).
-
Racemic ketamine’s impact on functional brain network connectivity in major depressive disorder (MDD) may offer valuable insights into its mechanism of action in mood disorders. CHDR utilised repeated resting-state functional magnetic resonance imaging (rs-fMRI) measurements to explore time-sensitive changes in functional brain connectivity following racemic ketamine administration in MDD patients (read more here). Connections of interest (COIs) were based on the previously published corticolimbic-insular-striatal pallidal-thalamic (CLIPST) circuitry model of depression. Ketamine significantly reduced depression severity, with average MADRS total scores decreasing from 21 pre-dose to 10 24 hours post-dose. Alongside this, significant rs-fMRI connectivity changes were observed in MDD-related COIs as defined by the CLIPST model, but no significant changes occured in COIs not previously associated with MDD.
This study demonstrated that racemic ketamine specifically affects depression-related brain circuitry in MDD. Additionally, it underscores the value of analysing functional brain connectivity based on a neurocircuitry model of a specific CNS disease and drug action, offering a potentially novel approach in CNS drug development.
-
Brexanolone (BRX) was approved for the treatment of postpartum depression by the United States FDA in 2019. BRX is a neuroactive steroid and a potent positive allosteric modulator (PAM) of synaptic and extrasynaptic GABA-A receptors, enhancing both phasic and tonic central GABA-mediated inhibition through these respective mechanisms. Extrasynaptic GABA-A receptors are insensitive to the effects of benzodiazepines, as they lack the γ-subunits required for benzodiazepine binding, whereas neurosteroids may activate them by engaging other GABA-A receptor subunits that are necessary for the neurosteroid binding site. Pharmaco-electroencephalography (pEEG) has been demonstrated to be an informative translational methodology for characterising GABA-A receptor-mediated pharmacological modulation with various GABAergic compounds. Neurostimulation approaches, such as transcranial magnetic stimulation-evoked EMG (TMS-EMG) and EEG (TMS-EEG), may further elucidate the mechanism of action (MoA) of such compounds by demonstrating their effects on activated neurocircuitry, rather than under unstimulated conditions.
By combining TMS with EMG and EEG, CHDR characterised the pharmacodynamic profiles of BRX and the benzodiazepine lorazepam in terms of cortical excitability in healthy male participants. BRX dose-dependently increased not only the resting motor threshold (rMT) but also the long-interval intracortical inhibition (LICI), measured with paired-pulse TMS-EMG (pp-TMS-EMG) at an interstimulus interval (ISI) of 100 ms. In contrast, lorazepam showed a trend towards a reduced amplitude of the single-pulse motor evoked potential, in line with previous findings, but did not affect the pp-TMS-EMG endpoints. Furthermore, BRX demonstrated modulation of early TMS-evoked potential (TEP) components versus late TEP components, and an increase in frontal theta power versus a decrease in frontal theta power, representing a pharmacodynamic profile on both TMS-EEG and pEEG that distinguished it from lorazepam. Together, TMS combined with EMG and EEG clearly distinguished between combined synaptic-extrasynaptic GABA-A receptor target engagement by brexanolone and selective synaptic GABA-A receptor target engagement by lorazepam. More significantly, it supports the future application of circuit-based approaches using TMS to capture the unique pharmacodynamic properties of GABA-A receptor PAMs, which differ in terms of GABA-A receptor (subtype) target engagement.
-
CHDR developed two variations of touchscreen-based tapping tasks to quantify drug effects on motor symptoms associated with Parkinson's Disease (PD). Alternate Finger Tapping (AFT) involves tapping with the index and middle fingers between two closely placed targets, while Alternate Side Tapping (AST) requires tapping with the index finger between two targets placed on opposite sides of the screen. Each task assesses different aspects of motor activity: AFT focuses on fine finger movements, whereas AST also engages upper arm movement.
Methodological validation confirmed both tasks' repeatability and sensitivity, supporting their pharmacological validation in PD patients. After the withdrawal of dopaminergic agents, PD patients received either levodopa/carbidopa or a placebo in the off state. Levodopa/carbidopa significantly improved tapping speed, accuracy, and rhythm in AST. The effect sizes of AST were comparable to changes observed on the Movement Disorders Society - Unified Parkinson Disease Rating Scale (MDS-UPDRS). In contrast, while AFT was sensitive to levodopa/carbidopa in terms of improved tapping speed and accuracy, the effect sizes were smaller than both AST and MDS-UPDRS. Additionally, by employing machine-learning technologies, we developed an AST-based composite biomarker. This biomarker not only successfully detected levodopa/carbidopa effects and estimated PD symptom severity but also outperformed both tapping tasks and the MDS-UPDRS III in detecting treatment effects.
In summary, AST provides a rater-independent measure that is not only sensitive to dopaminergic drug effects in PD patients but also appears superior to the gold-standard, rater-based MDS-UPDRS III. As such, AST could serve as a pharmacological biomarker for novel dopamine-modulating drugs aimed at improving motor symptoms in PD.


