At CHDR, we specialise in studying the effects of immunologically active compounds across a range of models: in vitro, ex vivo and in vivo. Beyond routine laboratory tests, we utilise advanced molecular techniques such as immunophenotyping, cell sorting, RNA sequencing, proteomics and ex vivo culture systems to gain deeper insights into immune function. In each setting, we can assess a compound’s impact on basic physiology, or employ challenge models to induce an immune response, enabling us to evaluate the compound's effect on that response.
In vitro challenge models
At CHDR, we conduct in vitro studies using fresh human blood and tissue samples to assess the pharmacological activity of test compounds. We obtain these samples from both healthy individuals and patients through our strong collaborations with Leiden University Medical Center and other hospitals, ensuring reliable access. Once collected, the samples are used in controlled in vitro settings to evaluate compound efficacy.
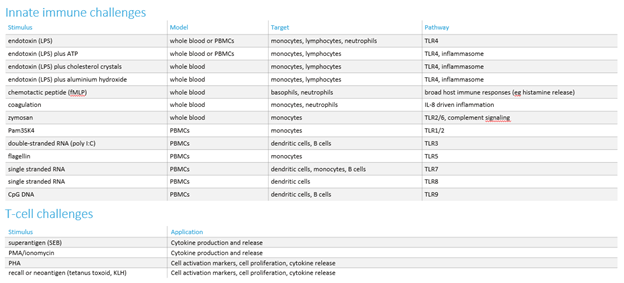
If we need to measure the impact of compounds on activated cells, we employ a challenge model to activate the relevant inflammatory pathway. We have developed a comprehensive range of these in vitro challenges (see the table below).
Ex vivo challenge models
We conduct ex vivo studies using fresh blood and/or tissue samples obtained from subjects who have received an investigational compound, placebo, or an active control. These samples are then used to assess the impact of new compounds on a broad spectrum of targets and pathways.
As for the in vitro studies, we offer a range of ex vivo challenges to evaluate the effects of new compounds (see table below). Additionally, we have the capability to develop customised ex vivo challenges, specifically designed to investigate the mechanism of action of new test compounds.
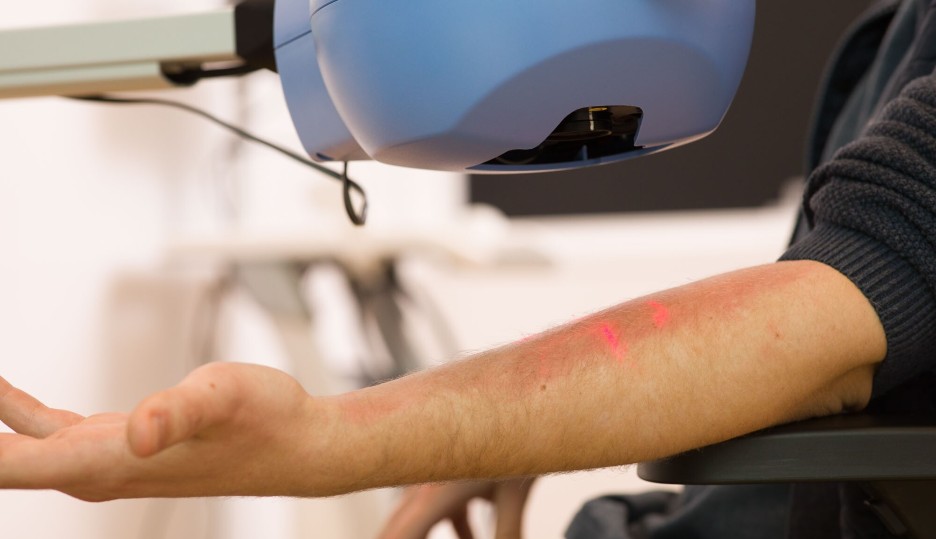
In vivo challenge models
At CHDR, we use in vivo challenge models to study the effect of new investigational compounds on local and/or systemic immune responses, both innate and adaptive. For local challenges, we inject a challenge agent into the skin or apply it to the skin, while for systemic effects, we administer it intravenously. We then assess the impact of the investigational compound on the immune response using different methods. Read more on our in vivo challenge models below.
In vivo challenge models
-
The intravenous lipopolysaccharide (LPS) challenge is a safe and effective method for obtaining valuable insights into the systemic effects of anti-inflammatory compounds in healthy subjects. The procedure involves administering either an investigational compound or placebo, followed by a precise dose of (GMP-grade) LPS directly into the bloodstream, which activates the innate immune system. This challenge allows us to assess the compound's impact on the immune response by measuring changes in peripheral blood markers, providing important data on the compound's ability to modulate systemic inflammation.
-
We use the intradermal LPS challenge to induce localised inflammation, providing a valuable model to assess the effectiveness of anti-inflammatory compounds. By injecting a small, controlled dose of LPS into the skin, we simulate an immune response similar to that of an infection or injury. This challenge activates the innate immune system, triggering the release of pro-inflammatory cytokines and other immune mediators, which cause redness, swelling, and pain at the injection site. To evaluate the inflammatory response, we employ non-invasive imaging techniques, such as laser speckle contrast imaging and multispectral imaging, or we collect biopsies or suction blisters for further examination. This approach allows for detailed assessment of the compound's ability to modulate tissue inflammation in real time, also covering vascular leakage.
-
We use the topical imiquimod challenge to trigger localised inflammation, offering an effective model for evaluating the efficacy of anti-inflammatory compounds. Imiquimod is a synthetic immune response modifier that stimulates the production of pro-inflammatory cytokines by activating the toll-like receptor 7 (TLR7) pathway. When applied topically to the skin, it triggers a potent immune response, mimicking conditions like viral infections or inflammatory skin diseases. We monitor the resulting inflammation through non-invasive imaging techniques, such as laser speckle contrast imaging and multispectral imaging, or by collecting and analysing skin biopsies, allowing for detailed assessment of the compound’s ability to reduce or modulate the immune response in the affected area.
-
We use the Keyhole Limpet Hemocyanin (KLH) challenge to assess adaptive immune responses. KLH drives a humoral and a cellular response, so the model is applicable for the pharmacodynamic evaluation of a wide range of immune-targeted drugs, modulating processes such as T cell and B cell activity, antigen presentation, and support by innate immune responses.
At CHDR, the KLH challenge is conducted using a two-step process to assess both systemic and local immune responses. Healthy volunteers are first vaccinated with low molecular weight KLH, a registered antigen (Immucothel®), commonly used in clinical practice. The immune response is then evaluated by quantifying KLH-specific antibodies in the circulation.
A few weeks after vaccination, a low dose of KLH is injected intradermally to stimulate a local inflammatory response. This response is carefully monitored and quantified using multispectral imaging (to look at redness) and Laser Speckle Contrast Imaging (to look at perfusion), combined with invasive sampling (biopsies, blisters) for molecular and cellular evaluations. This dual approach allows for a comprehensive evaluation of both the systemic and local immune responses to the KLH antigen.