Capabilities and solutions
At CHDR, we take a multi-faceted approach to pharmacology and dermatology. We investigate treatments for dermatological conditions such as psoriasis, eczema, allergic reactions, and inflammation, utilising advanced methods and biomarkers to objectively monitor clinical responses. Through these approaches, we can measure the effects of transdermal and intradermal drugs on the skin. Additionally, we study systemic conditions by evaluating skin responses, for example by measuring nerve fibre conduction, surface blood vessels, and local inflammation. Moreover, we are actively exploring ways to enhance wound healing.
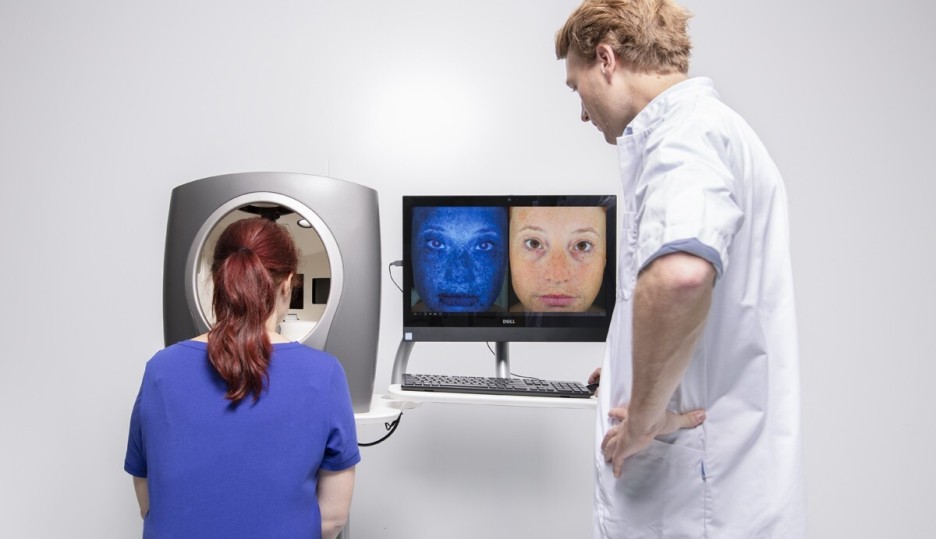
The DermaToolbox
CHDR's DermaToolbox is a comprehensive battery of objective measurement tools designed to systematically quantify symptoms and assess the effects of new dermatological treatments. It includes a diverse range of advanced methods, such as Transepidermal Water Loss (TEWL), Laser Speckle Contrast Imaging (LSCI), Optical Coherence Tomography (OCT), Multispectral Imaging and high-definition 3D stereophotography. These techniques enable precise measurement of skin lesion dimensions, properties, and surface features.
Application of inflammatory challenges for dermatology
We have successfully validated and applied inflammatory challenges in clinical experiments, contributing to the effective early development of multiple investigational products within CHDR's clinical studies. Our reliable and accurate methodologies induce inflammatory responses both in vitro (using whole blood or isolated immune cells) and in vivo. This approach enables the evaluation of immune-modulating effects of new drugs at the earliest stages of development.
Recruitment for dermatology trials
At CHDR, we conduct studies across various medical conditions. By working closely with medical centers, we enhance our ability to recruit a diverse pool of patients. Our dedicated recruitment team, supported by these strong collaborations, ensures a consistent and efficient participant flow for each study. This integrated approach allows us to tailor our recruitment strategies to the specific needs of each clinical trial. Our expertise spans a variety of conditions, including but not limited to:
Atopic dermatitis
15 trials with more than 1000 patients included
Plaque psoriasis and psoriatic arthritis
8 trials with more than 300 patients included
Cutaneous T-cell lymphoma (CTCL)
10 trials with more than 250 patients included
Urticaria
6 trials with more than 100 inclusions
Cutaneous lupus erythematosus (CLE)
5 trials
Hidradenitis suppurativa (HS)
2 trials
Vitiligo
HPV-induced disorders (10 trials)
Healthy volunteers (30+ trials)
Pharmacological and trauma-based challenges of the skin
First in human trials
If possible, studies in patients are conducted on an outpatient basis. While patients visit our facilities for selection and follow-up appointments, the trial itself is conducted off-site, allowing patients to remain at home and continue their daily activities. This approach improves recruitment rates and ensures a more natural and real-world assessment of treatment effects.
Clinical Network for Trials in Dermatology
We regularly conduct clinical trials in close collaboration with the Clinical Network for Trials in Dermatology (CONNECTED), a dynamic network of dermatologists with a shared passion for science and education. This expanding network now includes over 170 dermatologists from academic hospitals and peripheral clinics, with new members continually joining.
Our partnership with CONNECTED not only provides access to key opinion leaders in dermatology, but also enables rapid patient recruitment through referring dermatologists.
Hospitals involved in the network include, but are not limited to:
Leiden University Medical Center (LUMC)
Erasmus University Medical Center, Rotterdam
Amsterdam University Medical Centers (AUMCs)
Utrecht University Medical Center
Unlocking the Potential of OX40L Inhibition: From Early Safety to Breakthrough Atopic Dermatitis Treatment
Activation of the OX40‐OX40L pathway possibly contributes to resistance of T lymphocytes to regulatory signals and, therefore, OX40‐OX40L signaling may be a target for the treatment of auto‐immune diseases. Recently, we conducted a first-in-human trial of an anti-OX40L monoclonal antibody. The primary goal was to assess the safety, tolerability, and pharmacological activity of the drug, but we also explored the pharmacodynamic effects using a keyhole limpet hemocyanin (KLH) challenge model combined with advanced skin measurements. We showed that the drug was safe and well‐tolerated, and we demonstrated proof‐of‐pharmacology, as the drug suppressed the KLH‐driven neoantigen immune response via OX40‐OX40L signaling interference.
Encouraged by these results, the sponsor advanced the drug into a Phase 2b study, specifically targeting atopic dermatitis. The Phase 2b trial demonstrated impressive results, with an 80% improvement in atopic dermatitis symptoms, positioning the drug as a potential best-in-class treatment. Following the success of this trial, the company developing the drug was acquired by a major pharmaceutical corporation, further validating the compound's promising clinical profile and its potential impact on the treatment of immune-mediated conditions.
This case highlights the added value of including a pharmacological challenge model, and in this case specifically our KLH challenge model, in an early phase clinical trial.
Practical answers to important research questions
-
Early in drug development, CHDR can collect a wealth of data regarding the compound’s intended and/or unintended effects, even in healthy subjects. This data-intensive approach facilitates rational decision-making regarding the course of drug development. In dermatology 3D photography, total body mapping of skin lesions, objective lesion quantification and other innovative measurements can serve as robust predictors of clinical efficacy.
-
Pharmacokinetics is one of CHDR’s main areas of expertise, and CHDR researchers study systemic absorption in the initial clinical phases of drug development. This proactive approach allows researchers to minimise the risk of adverse systemic effects in subsequent trials, which is particularly important when patients apply the compound at home.
-
In recent years, CHDR has selected and validated a comprehensive panel of biomarkers for measuring inflammation ex vivo. In addition, we have developed several pharmacological and mechanical challenge models to study inflammation. Using these approaches, we can measure the effects of both systemic and topical drugs against allergy and/or inflammation in healthy volunteers.