Summary
CHDR maintains a large, up-to-date database of over 50,000 active study participants, including both healthy volunteers and patients. Besides our dedicated recruitment website (proefpersoon.nl), we actively reach out to potential study participants in a range of ways – from sponsoring sporting events and concerts, to advertising in print and online media. CHDR is ideally situated to recruit specific patient populations, which we do through direct contact, as well as via patient organisations and physicians.
We value the vital contribution that our study participants make to research. We are proud to offer not only state-of-the-art research facilities but also top-notch subject accommodation. Many subjects who have participated in a trial return to CHDR for additional studies.
Recruiting healthy volunteers
As soon as the Medical Ethics Committee gives a new protocol the green light, our dedicated recruiters reach out to potential subjects by phone and/or email. This approach is often sufficient to reach the number of subjects required to complete the study. Where necessary, we supplement our usual recruitment channels by placing ads in local newspapers as well as online on Facebook and in the form of website banner ads.
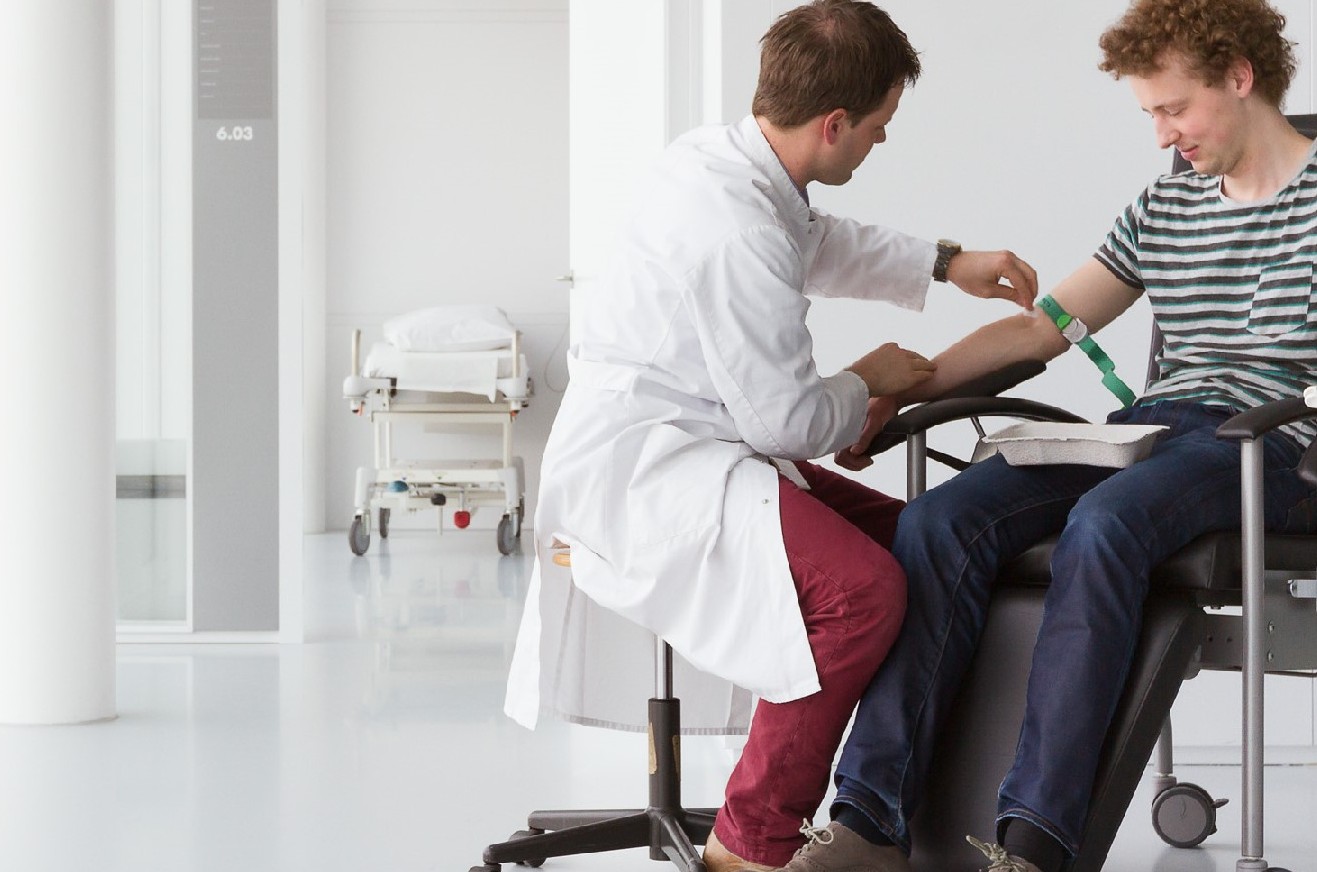
In the next stage of the recruitment process, interested volunteers are contacted personally by a recruiter to provide them with important additional information regarding the study. Prospective participants are then invited to CHDR for an information meeting, where they are given all the details they need in order to make a clear, informed decision regarding whether they wish to participate. Subjects who provide written informed consent are then invited for medical screening (in some cases, the information meeting and medical screening can be combined in a single visit). Medical screening can also be used to train subjects to perform specific tests, for example if the study will include the NeuroCart® or PainCart® test batteries.
Recruiting for patient studies
CHDR increasingly performs studies involving patients with specific diseases or other medical conditions. Although recruiting patients is generally more difficult than recruiting healthy volunteers, we are able to overcome these challenges thanks to our creative and diverse recruitment strategies. One pillar of this approach is the strong relationships we maintain with various patient organisations. In the Netherlands, most patient advocacy groups are keenly interested in participating in innovative scientific research, especially given that these groups represent the end-users of that research – the patients themselves. To reach these patient groups, CHDR places advertisements in the newsletters and on the websites of these patient organisations, who also support the effort by alerting specific patient groups. Depending on the specific patient population needed for a study, adverts can also be placed in other media outlets. In addition to these direct forms of recruitment, patients are often referred to CHDR by our vast network of clinicians in a variety of fields. Together, these strategies help ensure the smooth and timely recruitment of suitable subjects for every patient study conducted at CHDR.
Ready-for-Research
Rather than recruiting patients on an as-needed basis, the Ready-for-Research approach establishes a pool of well-defined patient groups who are ready and willing to participate in new trials, thereby significantly reducing timelines for recruitment and screening.
First, we recruit patients who have specific medical conditions and are interested in participating in drug studies. We then screen these patients using specific protocols that have been approved by an ethics committee. After providing written informed consent and a full medical history, patients receive a comprehensive physical examination at our main research facility, including blood work and performance on test batteries (for example, NeuroCart®). For the patients, this experience is similar to visiting an outpatient clinic, and we do our utmost to make them as comfortable as possible. Depending on their specific medical condition and disease stage, patients can return to CHDR on a regular basis (for example, once or twice a year) for re-screening in order to assess their condition, general health, and other possible factors that may affect their ability to participate in an upcoming study.